Medical wearable devices could be defined as biosensors that can monitor the physiological data of a patient. Theydo so with the aid of a wireless or remote communication. Similarly, it is any form of a wearable item that can be attached to the body. Some good examples include smart clothing, activity monitors, patches and smart watches.
Basis Science, which is among the leading manufacturers of top-notch fitness devices, has stopped the sales of its latest wearable device. The reason for the stop is the high number of overheating concerns. Apparently, their newest wearable device has been overheating and users have been complaining a lot. For this reason, the company will not sell any more of these devices. In addition, it has requested users who already have the wearable device to stop wearing it for their safety. It has promised users of a software update that will be in a position to solve the problem of overheating once and for all. Until then, do not use the wearable device if you already own one.
A good number of wearable medical gadgets are mostly concerned with exercises including jogging, walking or other types of exercises including muscle activity. Others measure calories burned, distance travelled, heart rate and so much more. Most include GPS tracking and monitoring as a way of enhancing accuracy in the measurement of distance covered. Other wearable medical devices encompass different physiological measurements and it is expected that future devices may incorporate enhanced functionalities such as monitoring brain activity, blood pressure, temperature, posture, pain relief and so much more.
It is very normal to hear that new wearable gadgets are having problems. The truth is, even big names in the industry such as Jawbone and Fitbit have had similar problems in the past. For instance, Jawbone had to shut down without notice. However, it had to offer a refund to its users for its original band return. Similarly, Fitbit had to offer its users with voluntary recalls on Fitbit Force. This is because most users frequently complained about skin rashes.
In addition to the concerns on wearable devices, there are more concerns relating to health and digital technology. There are numerous concerns about the lack of effective ways of enriching the relationship between patients and physicians. This has led to medical related associations such as the Medical Association of America, to warn people against some possible digital health threats. Apps do not provide users with quality patient safety and this is a major concern. In addition, the electronic health records do not allow physicians to effectively communicate with their patients in the best ways possible in order to provide them with proper ways of undergoing treatments.

It is sad to note that, the industry is evolving at a slow pace from ineffective digital health records. For this reason, there is need to develop high quality apps as well as digital health products. The AMA is aiming to advocate for these concerns. In addition, it is looking forward to work closely with the industry so that it can test and act as a medical trial for wearable manufactures that are looking to develop new devices for physician use.
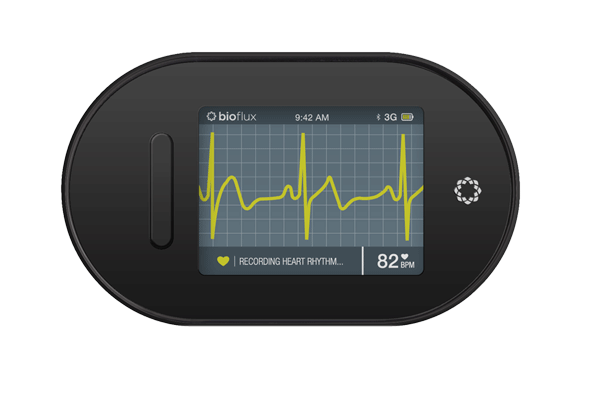
A good number of companies are working hard to develop technologies that can overcome such issues. For example, the OTCMKTS: BTCY BiotricityInc is currently developing wearable devices that are excellent in health management. In the meantime, the company is developing two devices that are expected to be available in the consumer market late this year. These are the Bioflux, which is wearable device that can monitor rhythm and connects to a renowned ECG (Electrocardiogram). In addition, it is expected to have a software component with FDA clearance. The second product is the Biolife, which is well crafted to provide lifestyle and health solutions. It will be in a position to monitor ECG, calories, physical activity, respiration rate, temperature and much more. With time, this type of technology is expected to become more mainstream. This will allow for proper treatments. The best part is that patients and their doctors will be in a position to use the wearable medical devices effectively and maintain long distance relationships.
Another major concern with wearable medical devices is the battery problem. There have been a lot of concerns about industry regulation, testing and manufacturers. The industry has witnessed a tremendous growth rate over the last few years and many manufacturers have started taking shortcuts by developing gray market products and illegal batteries that do not have the necessary circuits for protection. Necessary protection circuits prevent Li-ion based batteries from overheating, catching fire or overcharging. This has led to most companies to start taking measures aimed at protecting their intellectual properties rights against copycat or fake batteries. For example, some companies have introduced a number of security features including holograms and invisible inks. These are aimed at helping to eliminate fakes and ensure batteries used in wearable medical devices are authorized and original.
There is need for clinical medical gadgets to be reliable and highly accurate when it comes to times recording. This makes it necessary to develop micro-batteries that can last longer. Information processing systems should be perfectly integrated in medical devices to enable them take care of large amounts of patient data.
See Also: The Next Phase of Tracking Health, Happiness and Fitness
Conclusion
Although the healthcare technology has continued to show positive growth, most hospitals still have similar IT budgets. For this reason, it is important for executives in health IT to make informed decisions about the technologies that are worth financial investments and time. This will enable them choose the right wearable medical devices and technology for implementation. There are numerous guides online to help health IT executives with the same.





No Comments so far
Jump into a conversationNo Comments Yet!
You can be the one to start a conversation.